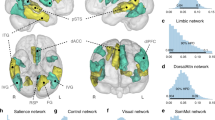
Loneliness is associated with increased morbidity and mortality. Deeper understanding of neurobiological mechanisms underlying loneliness is needed to identify potential intervention targets. We did not find any systematic review of neurobiology of loneliness. Using MEDLINE and PsycINFO online databases, we conducted a search for peer-reviewed publications examining loneliness and neurobiology. We identified 41 studies (n = 16,771 participants) that had employed various methods including computer tomography (CT), structural magnetic resonance imaging (MRI), functional MRI (fMRI), electroencephalography (EEG), diffusion tensor imaging (DTI), single-photon emission computed tomography (SPECT), positron emission tomography (PET), and post-mortem brain tissue RNA analysis or pathological analysis. Our synthesis of the published findings shows abnormal structure (gray matter volume or white matter integrity) and/or activity (response to pleasant versus stressful images in social versus nonsocial contexts) in the prefrontal cortex (especially medial and dorsolateral), insula (particularly anterior), amygdala, hippocampus, and posterior superior temporal cortex. The findings related to ventral striatum and cerebellum were mixed. fMRI studies reported links between loneliness and differential activation of attentional networks, visual networks, and default mode network. Loneliness was also related to biological markers associated with Alzheimer’s disease (e.g., amyloid and tau burden). Although the published investigations have limitations, this review suggests relationships of loneliness with altered structure and function in specific brain regions and networks. We found a notable overlap in the regions involved in loneliness and compassion, the two personality traits that are inversely correlated in previous studies. We have offered recommendations for future research studies of neurobiology of loneliness.

Loneliness is a critical determinant of well-being and also a grand challenge to society [1, 2]. Defined as distress due to perceived discrepancy between desired and existing social relationships, loneliness is associated with higher rates of cardiovascular disorders [3], dementia [4], anxiety, depression, suicidal ideation [5, 6], and 30% greater mortality [7,8,9]. Loneliness is distinct from objective social isolation or the lack of social relationships/contacts. The National Academies of Science, Engineering, and Medicine recently published a report on social isolation and loneliness among older adults, calling for more research of neurobiology and interventions [2]. During the COVID-19 pandemic, loneliness, which has been linked to physical distancing measures, is a growing concern for all age groups across the world.
Humans are a social species and have ingrained neural, hormonal, and genetic mechanisms to help navigate social connections. Absence of quality relationships threatens health and reproduction [10]. Cacioppo et al. posited loneliness evolved to improve survivability when socially isolated, through hypervigilance and increasing motivation to connect with others [10]. Animal models of social isolation have demonstrated alterations in neurotransmitters, receptor sensitivities, and levels of certain biomarkers [10, 11]. Few studies have examined the impact of social isolation on specific brain regions [11,12,13]. Furthermore, the subjective nature of loneliness as well as inter-species differences in social functioning and brain structure limit the applicability of the animal studies to the uniquely human state of loneliness [11].
Our recent investigations have found a strong and consistent inverse correlation between the personality traits of loneliness and wisdom, especially the empathy/compassion component of wisdom [14,15,16,17]. In contrast to loneliness, wisdom is associated with better mental and physical health [18,19,20]. The prefrontal cortex and limbic striatum reportedly play a major role in the neurobiology of empathy/compassion and wisdom [21]. Identifying neurobiological mechanisms underlying loneliness is critical for understanding how loneliness contributes to poor mental and physical health and for conceptualizing potential pharmacological and neurostimulation targets. Therefore, we conducted a systematic review to identify and synthesize published brain-based findings linked to loneliness.
We conducted a literature search for peer-reviewed publications examining loneliness and neurobiology, outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram (Fig. 1). We surveyed MEDLINE and PsycINFO online databases on September 24, 2020, with the following inclusion criteria: (1) use of a validated scale for assessing loneliness and a measure of neurobiology, (2) published in English, (3) minimum of 10 human participants, and (4) statistical analysis examining the relationship of loneliness and neurobiology. We excluded animal studies and literature reviews.
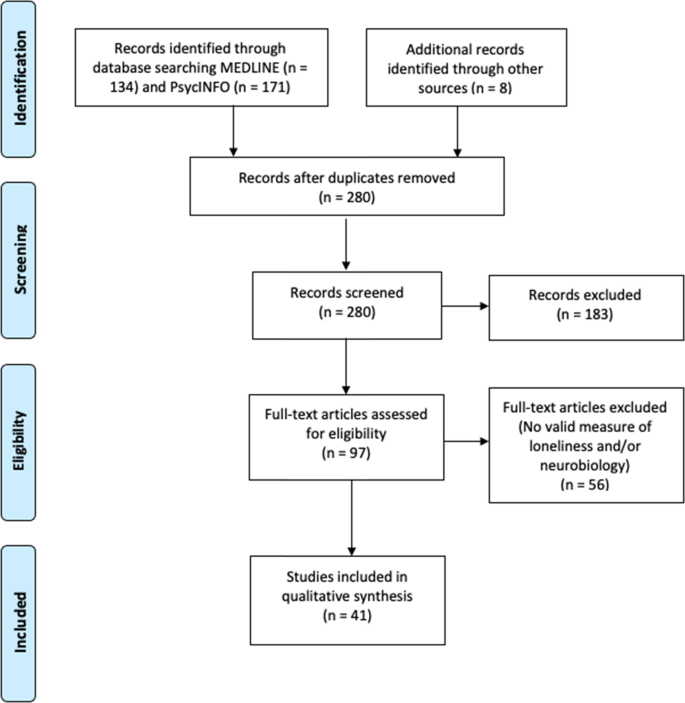
We defined validated measures of loneliness as scales or questions that measured feeling lonely, socially isolated, or disconnected. The most commonly used scale was the University of California Los Angeles Loneliness Scale (UCLA-LS [15, 17, 22]), although we also included validated, briefer multiple- or single-item questions [23]. Neurobiology measures included assessments of brain structure or function: computed tomography (CT), magnetic resonance imaging (MRI), functional MRI (fMRI), diffusion tensor imaging (DTI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), or electroencephalography (EEG). We also included brain pathology studies and genetic investigations that extracted genetic materials from brain regions. We did not include studies with only cognitive measures or studies using cortisol or other peripheral biomarkers from blood or other tissues outside the brain. The specific search strategy is outlined in Supplementary Appendix A.
The search yielded 305 articles of interest. After removing duplicates and adding potentially relevant papers from bibliographies of the articles selected, each study title and abstract was screened for eligibility by at least two authors (JAL, ERM, KEY, MR). Articles with any uncertainties were discussed and resolved among all authors. Data from each of the final batch of 41 studies selected (Fig. 1) were extracted by the primary author and checked by at least one other coauthor. Sample sizes ranged from 19 to 10,129. Most of the studies (61%) included fewer than 100 individuals, 38% with 100–942 individuals, and one study with over 10,000 individuals.
To assess the quality of the studies, we used the Joanna Briggs Institute appraisal checklist for cross-sectional studies and cohort studies and Newcastle-Ottawa Scale for case-control studies (Supplementary Table 2).
Twenty-four studies focused on younger adults (mean age 18–60; [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]), 12 on older adults (mean age >60; [48,49,50,51,52,53,54,55,56,57,58,59]), two on adolescents [60, 61], and two across the lifespan [62, 63]. Most reports included healthy individuals while seven focused on clinical populations: four with depression [47, 49, 51, 57], and one each with traumatic brain injury (TBI) [58], schizophrenia [28], and severe hearing impairment [27]. Eighteen studies came from the US, 13 from China, three each from Germany and Taiwan, and two from the UK, and one each from Japan and the Netherlands. Of the 41 studies, 5 (12.2%) had hypothesis-driven analyses (e.g., region of interest focused), 21 (51.2%) had exploratory analyses (e.g., whole brain analyses), 10 (24.4%) had both, with 5 studies not fitting into any of the above categories.
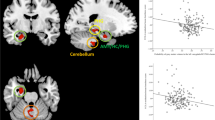
Fifteen studies analyzed the relationship between loneliness and brain structures using CT [58] or MRI. Twelve were cross-sectional, with eight focusing on gray matter volume, [26, 31, 35, 36, 46, 57, 59, 63], and four on white matter features, employing DTI or diffusion MRI [29, 32, 38, 61]. The three longitudinal investigations included a randomized controlled trial (RCT) of effects of exercise on gray matter volume [54], a prospective cohort study of progression of white matter hyperintensities [53], and a study of TBIs localized to different brain areas [58]. These study findings are summarized in Table 1.
While there is no literature on loneliness in non-human animals, neurobiological correlates of social isolation have been examined in several animal studies and a few human studies. There are a few reviews focused on integrating animal social isolation and human research [11,12,13]. However, the social isolation literature in animal models focus more on changes in endocrinology, neurotransmitters, and oxidative stress, rather than neuroanatomical or functional brain differences [11]. These differing methodologies and paradigms make direct comparison challenging, though there are likely overlapping risk and protective factors for social isolation and loneliness.
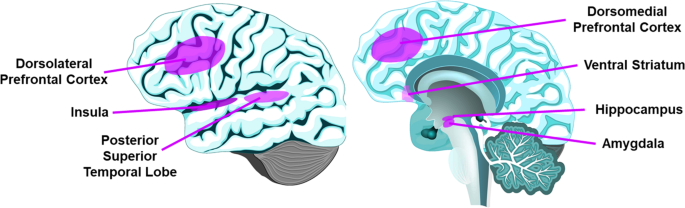
The PFC mediates higher-order behaviors like emotional regulation and inhibitory control [65, 66]. The dlPFC is implicated in working memory and executive function [67], and mPFC is implicated in self-referential processes such as self-criticism in social situations [68]. All 14 imaging studies examining PFC found associations of loneliness with structural (gray matter volume and white matter integrity) or functional components (activation with social vs. nonsocial images, and functional connectivity). These results are consistent with a previous review of animal studies of social isolation implicating PFC [13], and support loneliness as a complex socioemotional trait.
The insula somatic marker hypothesis states that insula receives and integrates information to create a “global emotional moment” [69]. Anterior insula plays a role in various behaviors including emotions, pain, and self-awareness [70]. In studies of gray matter, white matter connectivity, task-based activation, insula was reportedly associated with loneliness [25, 28, 29, 32, 58, 59]. It has been proposed that social rejection activates similar regions as physical pain, as supported by bilateral anterior insula activation with feelings of loneliness [70, 71], although a more recent social rejection meta-analysis of fMRI studies did not find anterior insula involvement [72].
The amygdala is implicated in fear detection, positive stimuli processing, and emotional memories [73]. Four studies of amygdala reported some association between gray matter volume or task-based activation and loneliness, with possible age- and sex-interactions [36, 46, 54, 59]. These findings are consistent with loneliness activating brain regions that support experiencing emotions. However, an fMRI study found no relationship with amygdala response to social stimuli [62].
The ventral striatum, which includes nucleus accumbens, plays a central role in reward reinforcement [74]. Three studies of ventral striatum response to social images produced divergent results [25, 34, 62]. A recently published fMRI study (published past our cutoff date) reported similar activation in substantia nigra/ventral tegmental area (SN/VTA) among young adults undergoing either 10 h of social isolation or fasting from food [75], supporting loneliness as a state that motivates one to seek social interaction, much like hunger motivates one to seek food. Interestingly, lonely participants had less activation in the SN/VTA. Research based on social isolation in rodent models and social rejection in human experiments indicates that social isolation may alter social approach motivation [13], consistent with the findings that loneliness differentially alters ventral striatum and structures related to reward pathway.
The posterior superior temporal cortex, implicated in social cognition [76], was associated with loneliness in four studies [25, 26, 32, 61].
The hippocampus, known for its role in memory [77], and the cerebellum, known for sensorimotor coordination as well as cognitive and affective processes [78, 79] each had three papers that associated their function or structure with loneliness.
Attentional networks are responsible for effortful versus environmental, stimulus-driven control of attention, and are localized to distinct anatomical areas with specific cognitive functions [80]. Four publications reported that loneliness was associated with differences in ventral attentional (including TPJ), dorsal attentional, and cingulo-opercular networks, in terms of functional and effective connectivity [29, 37, 39, 43]. Attentional networks may be linked to hypervigilance and stress reactivity that are putatively involved in loneliness.
Visual systems are responsible for processing visual information. Five studies reported associations of loneliness with differences in primary and secondary visual cortex in terms of volume [46], functional connectivity [43], causal flow [39], or activation with social images [25, 63], supporting Cacioppo et al.’s hypervigilance theory of loneliness [6].
The DMN is active when the human brain is at rest and is implicated in mental representations of self across time and space, theory of mind, and pro-social behaviors [81]. Three studies showed an association between loneliness with DMN functional connectivity [43, 47, 51]. One report noted more dense, less modular connections between attentional, visual, and DM networks in lonely persons. A recent large (n = ~40,000), multi-modal study (published after our cutoff date for inclusion in this review) reported increased volume and white matter structural connectivity as well as increased functional connectivity of the DMN in lonely individuals [82]. Together, these results suggest that higher-order brain regions localized to PFC and DMN may play a critical role in loneliness. The DMN may be differentially activated when we are thinking about others; however, dysregulated activity in DMN may contribute to rumination and negative feelings associated with loneliness.
Two EEG studies showed that lonely individuals had faster ERPs to negative or threatening stimuli [30, 33], consistent with the hypervigilance hypothesis of loneliness [6], while another report found no difference in ERPs with a nostalgia-related task [64].
Regarding AD markers, two PET studies reported greater amyloid and tau burden [50, 56], and one MRI study reported progressive increase in white matter hyperintensities among lonely older adults [53]. However, one post-mortem study found no such differences in plaques, tangles, or infarcts [48]. Two studies extracting RNA from the brain identified differential AD-related gene expression in lonely individuals [52, 55]. The overall findings related to AD align with meta-analytic evidence linking loneliness to increased risk of AD [83].
The brain regions highlighted in this review of loneliness may also have roles in other related constructs. For example, we have found a strong inverse correlation between loneliness and wisdom, especially its compassion component [15,16,17]. An overview of the neurobiology of wisdom has highlighted the major roles of PFC, especially dlPFC, vmPFC, anterior cingulate, and insula as well as amygdala [21, 84]. One MRI study reported that loneliness and empathy were inversely associated with white matter density in lateral PFC, insula, and TPJ [32], while another MRI study found no links with gray matter density [26]. A recent EEG study demonstrated that loneliness and wisdom/compassion were related to contrasting modulations of cognitive processes, invoking similar (TPJ) and distinct (superior parietal vs. insula, respectively) neural circuits in specific emotional contexts [85]. These relationships are correlational and warrant further study employing neurobiological perturbations.
This review article as well as the included studies have limitations. It is possible that, despite our best efforts, we missed a few relevant papers. Also, we did not include articles in non-English languages, and 73% (30/41) of the reports came from the USA or China, thereby limiting the generalizability to other countries. Most investigations were cross-sectional, preventing causal inferences. There may be confounding factors that are driving these relationships. There is risk of gender bias in self-reported assessments of loneliness. While there are no agreed upon objective measures of loneliness, indirect partial objective measures may include sedentary behavior assessed with wearable activity trackers, life space using GPS data, and sleep disturbances using wearable sensors. The studies included are limited by varied methodologies and analysis techniques in the rapidly evolving field of social neuroscience. For example, EEG has remarkable temporal resolution, but poor spatial resolution while the reverse is true with fMRI [86]. Many studies were hypothesis-generating and used single neurobiological modalities. Though one study included over 10,000 participants from the UK Biobank registry study [46], the majority (25/41; 61%) of the studies had fewer than 100 participants. Thus, most of the individual study findings are limited by small sample sizes, and overall generalizability may be low. Subject samples varied widely in sociodemographic characteristics, outcome measures, analysis protocols, and statistical methods, thereby precluding a metanalysis. It is not always clear if some brain regions not mentioned in the results had not been examined or were examined but not found to be significantly associated with loneliness.
Most studies only assessed and controlled for a small number of covariates such as demographic variables including age and sex. However, the complex psychosocial nature of loneliness extends beyond these basic demographic factors. Objective health status, environmental characteristics, stress, mental health, and personality traits are important confounders that were not included in many of the analyses. Only two studies had samples that could example the relationship of age (across the adult lifespan) with the loneliness-neurobiology associations [62, 63]. Wong et al. reported reduced cerebellar gray matter with older age, while D’Agostino et al.reported no age-related findings. While Düzel et al. presented age-related findings, they were restricted to older adults 61–82 years [59]. Several, but not all, studies have examined depression as a confounder [38, 58, 59], and three case-control studies specifically examined the effect of loneliness for the neurobiological differences between people with depression and healthy controls [45, 51, 57]. However, despite their potential impact, other constructs including grief, prolonged grief disorder, mild cognitive impairment, substance use disorders, and various stress-related conditions were not assessed and analyzed in most studies of loneliness.
This systemic review of neurobiology of loneliness identified how loneliness is linked to specific brain regions and networks, including PFC, insula, amygdala, hippocampus, attentional networks, and DMN, and a strong relationship with AD. However, researchers will need to replicate and expand the quantity and quality of these studies to understand the brain processes underlying loneliness. Moving forward, task-based neurocircuitry fMRI studies and multi-modal imaging studies have promise, due to the complexity of social cognition and functioning. These approaches would be well-suited to loneliness interventions to identify associated changes in connectivity. Future studies should also include large and diverse samples of well-characterized subjects followed longitudinally, with hypothesis-based approaches and appropriate multivariate statistical analyses, to examine the role of age and other relevant factors.
Studies should examine how the neurobiological findings are linked to other behaviors associated with loneliness—including sleep disturbances, sedentary behaviors, and limited life space. Assessments should include multi-modal assessments of social functioning—including use of social media, GPS-derived life space data, speech data, sleep, and ecological momentary assessments that examine loneliness as a state rather than a trait [87]. Neurobiological assessments that examine structural and functional integrity or harness neuromodulation techniques such as transcranial magnetic stimulation can also provide novel insights into brain alterations associated with loneliness. Furthermore, RCTs of novel loneliness interventions and associated neurobiological changes are warranted. Such research will pave the way for the development of therapeutic and preventive interventions to manage the behavioral pandemic of loneliness.
The authors acknowledge the help of Dr. Dilip Jeste in providing administrative support for the research effort as well as Ms. Paula Smith in preparing and submitting the manuscript.
This study was supported, in part, by the National Institute of Mental Health [NIMH R01MH094151 (PI: Dilip V. Jeste), NIMH K23MH119375-01 (PI: EEL), NIMH K23 MH118435 (PI: TTN)], by the VA San Diego Healthcare System, by the Stein Institute for Research on Aging (Director: Dilip V. Jeste, MD) at the University of California San Diego, and by IBM Research AI through the AI Horizons Network. The authors have no conflicts of interest with the work described.